During my NATURE-ETN PhD program, I had the chance to spend two months at the University of Reading, in the UK, supervised by Prof. Christine Cardin and Dr. James Hall.
Even if changing country and environment are always challenging, I realised how a great experience I had the chance to come across from the first moment I arrived in the UK. The first day I went to the lab, the group gave me a very warm welcome, and I felt at home from the beginning.
During this secondment, I learnt many new techniques and developed new skills, supervised by very professional and passionate scientists, always keen to teach me.
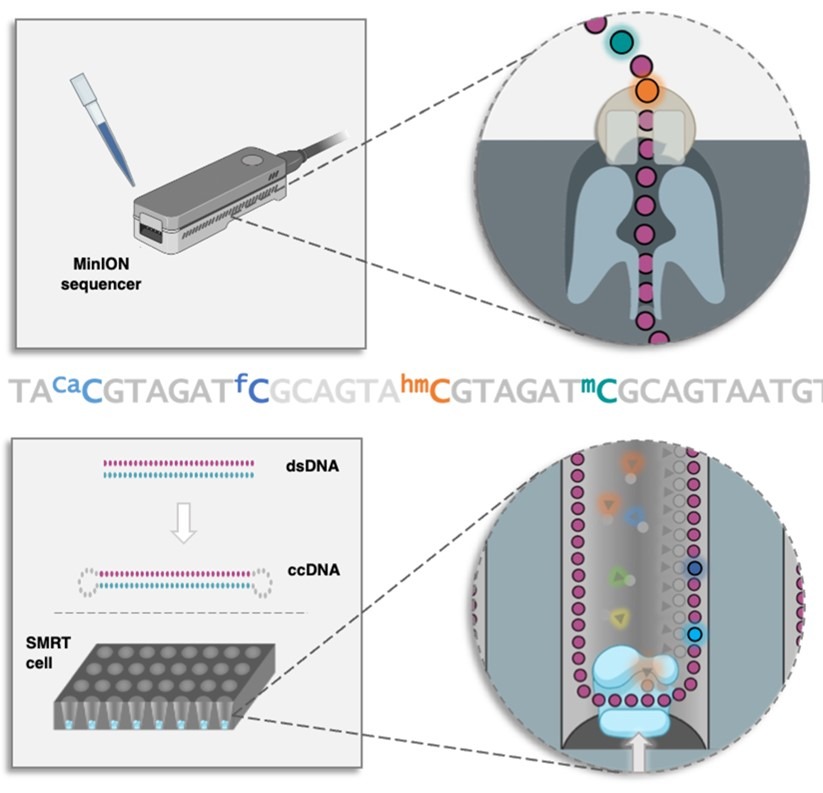
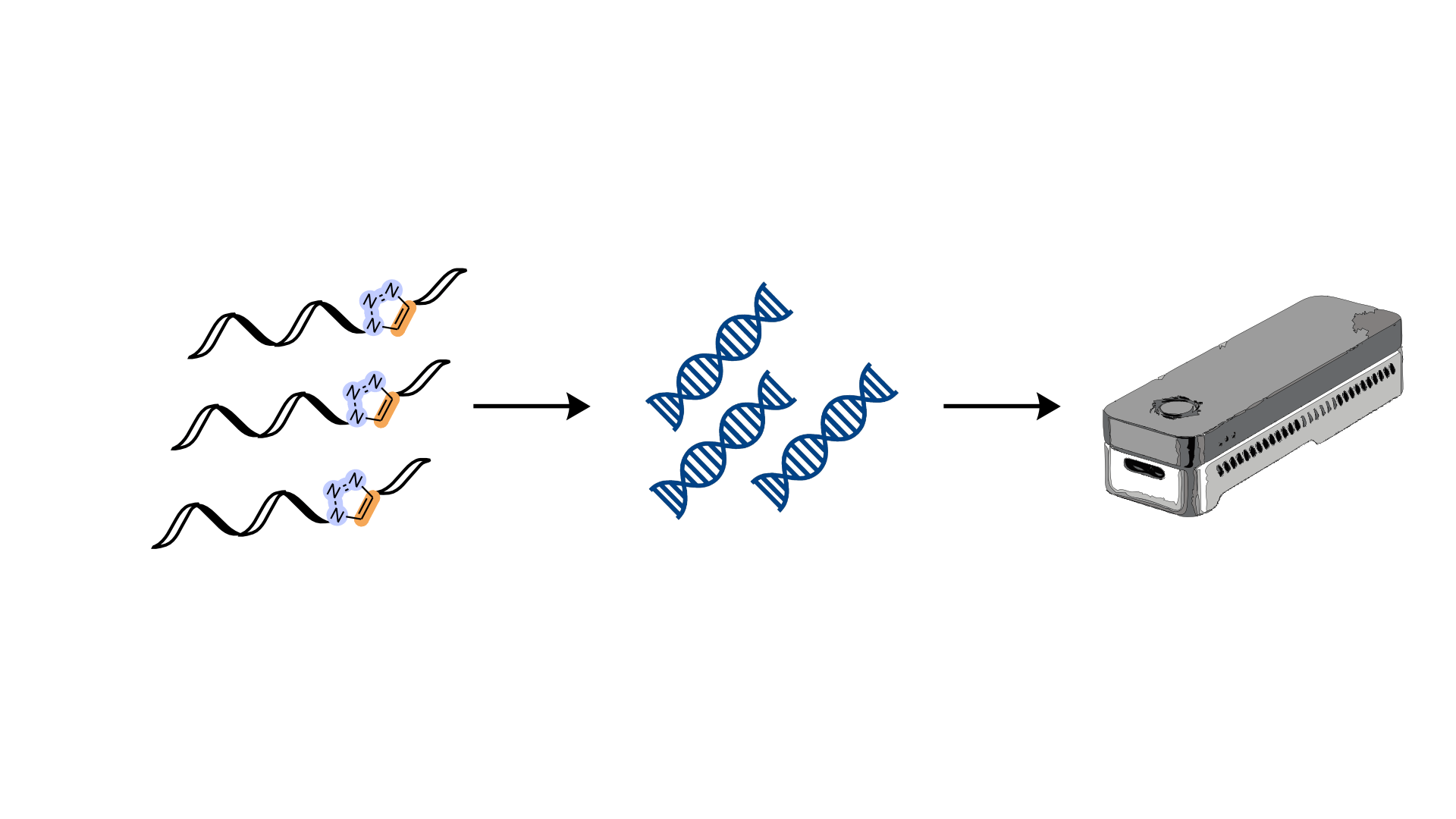
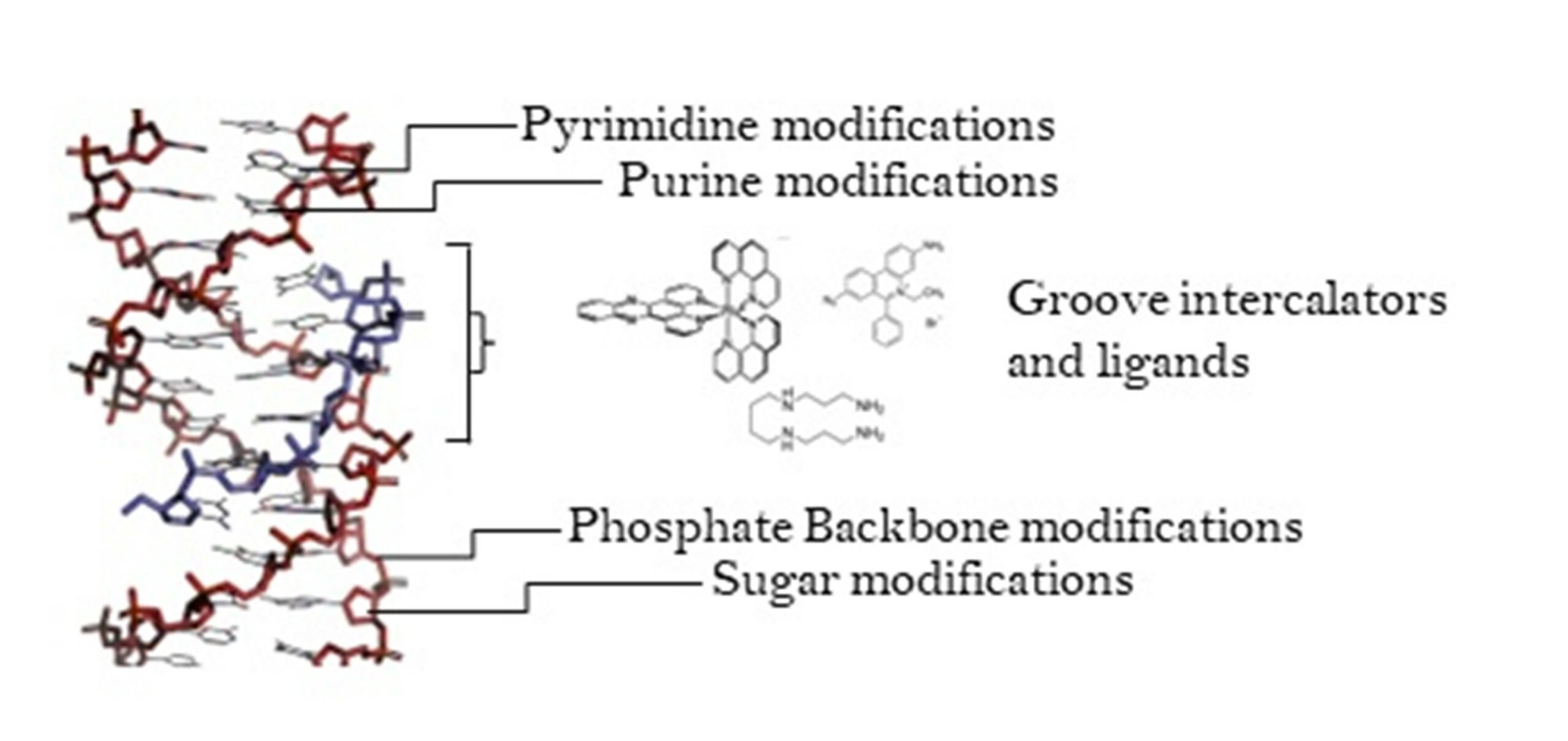
During these two months, I evaluated the interaction between the compounds I synthesised at Chimie ParisTech and the DNA, through thermal stability studies. Also, I learned how to use a chiral HPLC and crystallise the compounds and the DNA. These topics and techniques are really fascinating to me, and this experience helped me to better understand my project and its very important aim. Overall, during my secondment, I had the opportunity to learn, develop my scientific skills, and know a different scientific environment.
To be fair, that was not only work… I fell in love with the UK! During the weekend I visited many different places like London, Oxford, and the beautiful British countryside. I spent some evenings in the pubs drinking beers with my new friends and having tea with the delicious scones with clotted cream and jam (the “cream tea”) in the afternoon.
Also, we went out with the group for a beer a few times, as you can see in the picture.
This was a very great experience from both a professional and personal point of view, and I am grateful to be part of this fantastic ETN project.
By Maria Dalla Pozza (PSL)