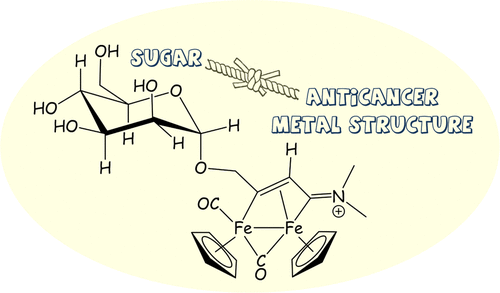
Recent collaborative work from NATURE-ETN and the University of Pisa has been published in Organometallics by ESR Maria Dalla Pozza from the group of Gilles Gasser at Chimie ParisTech, PSL University, CNRS, Institute of Chemistry for Life and Health Sciences (France), in collaboration with the team of Prof. Fabio Marchetti at the University of Pisa (Italy). The scientists investigated the anticancer activity of some di-iron compounds functionalised with sugar moieties to increase their cellular uptake and possibly their selectivity for cancer cells. The compounds, synthesised by Marchetti’s group, were tested against a panel of cancerous cells by ESR Maria Dalla Pozza.
Among the different categories of metal drug candidates, iron complexes based on the ferrocene scaffold have aroused notable interest. The antiproliferative activity of these compounds is ascribable to the redox chemistry of the ferrocenyl iron(II) center, which undergoes oxidation to Fe(III) in the tumour cells. This enhances the formation of toxic metabolites that lead to cell death. Di-iron compounds have been less investigated, however recent studies have disclosed a promising anticancer potential. In this study, the sugar functionalisation was performed on di-iron complexes to optimise the cytotoxic properties by exploiting the Warburg effect. Specifically, the anticancer activity of the compounds was evaluated using the resazurin assay and the scratch assay. Overall, this study pointed out that the antiproliferative activities of the new complexes correlate with their lipophilicity, ranging from moderate cytotoxicity to inactivity, and showing an absence of appreciable selectivity with respect to a non-cancerous cell line. On the other hand, analogous diiron complexes without the sugar moiety functionalisation, analysed as references, performed better in the same conditions. This confirms the potential of the present category of organometallics in the medicinal field.
Reference:
Schoch, S., Iacopini, D., Dalla Pozza, M., Di Pietro, S., Degano, I., Gasser, G., Di Bussolo, V., and Marchetti, F. Tethering Carbohydrates to the Vinyliminium Ligand of Antiproliferative Organometallic Diiron Complexes.
Organometallics (2022). https://doi.org/10.1021/acs.organomet.1c00519